What is Corrosion?
Corrosion is the event that they lose metallic properties by entering chemical or electrochemical reactions with the medium they are in metals. The most general sense is that materials are not able to be corrupted by environmental impact.
All kinds of material made from metal undergo less or very corrosion during use. All mechanical properties of corrosion metal changes, decreasing as corrosion and functionality progress corrosion. Steam boilers, petroleum and natural gas pipelines, nuclear reactors, bridges, deep well pipes, vessels, and all kinds of motor vehicles are fixed and working metallic parts, where corrosion is the most and has great danger. So that in any case as a big problem.
Damages caused by corrosion:
- Impact on human health
- Pollution
- The metallic materials produced are approximately 1/3 in the end of the year
How Does Corrosion Occur / Cause?
The environments of corrosion are generally moist or dirty air, saline, acidic or basic medium, alkaline environments. The anode, cathode, electrolyte (conductive solution) and the metallic transmission path must be found to realize the corrosion. Metal, the anode is oxidized, the metal ions are reduced to the oxygen on the cathode by passing the electrolysis. The electrons formed as a result of the oxidation of metal ions are transferred to the cathode with metal conductor. As a result, the reduction reaction occurs.
What Are The Kinds Of Corrosion?
Corrosion Types; It is in various ways according to the formation of the environment, the formation of the formation. The most encountered varieties are listed below.
- Uniform Corrosion (Homogeneous Distribution -Erable)
- Galvanic corrosion
- Corrosion of the pit
- Interval corrosion
- Chosen corrosion
- Corrosion between grains
- Corrosion with erosion
Uniform Corrosion (Homogeneous Distributed – Equal Distributed)
 The uniform corrosion is also called a homogeneous distribution or equal distribution corrosion. This type of corrosion is the most common type. In a particular order on a metal surface, the homogeneous distributed surface rust is called uniform corrosion. The steel and cast iron are formed on the air, if they come into water, water or soil in the atmosphere. Uniform corrosion is very low in aluminum materials or in alloys with aluminum in aluminum. At the beginning of the factors that cause uniform corrosion, the metal has contact with direct air, water or soil.
The uniform corrosion is also called a homogeneous distribution or equal distribution corrosion. This type of corrosion is the most common type. In a particular order on a metal surface, the homogeneous distributed surface rust is called uniform corrosion. The steel and cast iron are formed on the air, if they come into water, water or soil in the atmosphere. Uniform corrosion is very low in aluminum materials or in alloys with aluminum in aluminum. At the beginning of the factors that cause uniform corrosion, the metal has contact with direct air, water or soil.
Galvanic Corrosion
Corrosion type is called galvanic corrosion in which the materials that are interacting with each other and having different electrode potentials within the same medium in the same medium. While the risk of galvanic corrosion of the low-standard electrode potential is higher, the risk of galvanic corrosion is lower than the standard electrode potential is higher than the risk of galvanic corrosion. The liquid or moisture in the environment in which the metal is an electrolyte and this leads to the formation of galvanized corrosion cells. Especially in the ports of different metallic material pairs, galvanic corrosion is more common.

Pitting Corrosion
Some points of the metal surface are the type of forming the pit. In this type of corrosion, the potholes on the metal surface are increasingly growing and leads to the breakage of metal or to disappear completely in time. The pit corrosion has the power that can affect the most inner parts of the metal.
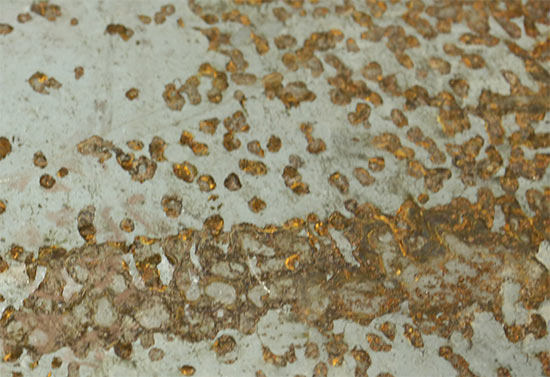
Interval Corrosion
Corrosion is the type of corrosion consisting of high corrosive substances in the slit and intervals during production or instructions on the metal surface. These slit and intervals are as clear enough to take any liquid to the surface but are narrow ranges enough to prevent it to flow.
Chosen Corrosion
Corrosion is not only in the structure of the metal, but both metal and elements in alloy metals may be corroded. Selective corrosion is the type of corrosion that causes the element and metal to move away from alloy in alloy metals.
Selective corrosion occurs in the most brass alloy metals. In brass alloys, selective corrosion occurs when the zone is away from the alloy of the zone. The frequency of chocolate corrosion in corrosion types is not very high.

Corrosion Between Grains
Metals are found in solid crystalline. Metal atoms are properly dispersed in this crystal structure. Iron and Steel are in Cubic-based crystal structure. Ostenitic stainless steels are superficially centered crystal structure. Crystal structures are effective in corrosion between the grains of metals. When the metals are dissolved, they solidify together in adjacent crystals when the cooling is abandoned. Grains of a large number of crystals are separated by boundary lines. In the narrow areas between grains, the crystal structure is irregular. These regions are where the metal is most resilient to corrosion.
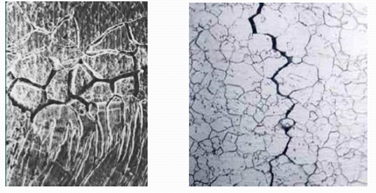
The inter-grain corrosion occurs because of any impurities between grains, for example, to have or not more of an alloy element. For example, a small amount of iron in aluminum may cause corrosion. Because the iron in the aluminum is very small soluble, so it is collected between grains.
Erosion Corrosion
If corrosive properties flow rapidly from the metal surface, erosion occurs next to the corrosion event. This corrosion type caused by a chemical interaction and mechanical wear is called erosion corrosion. Pipes are frequently encountered in transport systems using materials such as elbows, valves, pumps, propellers, mixers, condensers, turbine pallets and the most important factor affecting this type is the flow rate of fluid (flow). The erosion effect increases as the flow rate increases.

What Are The Methods Of Corrosion Prevention?
The measures to prevent corrosion of the metallic structure in a certain environment or to reduce wear rate are listed below.
- Selection of appropriate materials
- Alloy element added
- Heat treatment
- Proper design
- Cathodic protection
- The use of inhibitor
- Surface coating
- Anodic protection
Selection of appropriate materials
The most general way of preventing corrosion is the selection of suitable metal and alloys where it is used. In general, the stamina of pure metals are better than the metals containing the other elements, albeit with a small amount. Using pure metal in most applications, non-homogeneous parts are minimized and thus the corrosion of the pitch (pitting) is largely prevented.
Alloy element added
The corrosion resistance of some metals can be increased by adding alloy element. Some alloy elements increase the resistance of the material by creating or forming non-porous oxide films on the surface of the material.
Heat treatment
Segregation (decomposition) occur most of the cast parts. These parts are homogeneously homogeneous by implementing thermal operations such as homogenization, dissolution or stabilization, and thus increasing resistors.
Proper design
Designs to prevent accumulation of corrosive media (water etc) in the systems where corrosive substances are stored. In addition, very fine intervals should be avoided between the liquid deposit.
Cathodic protection
Cathodic protection is the most effective method used to protect metals from corrosion. Cathodic protection, when a clear current from the electrochemical cell passes a clear current, the anode oxidation reaction walks to the reduction reaction to be equivalent to the cathode. In such a system, there is no event of corrosion in the cathode region. Based on this theory, the anodic regions on the surface of a metal can be turned into the cathode and the corrosion event can be precisely prevented.
Anodic protection
Anodic protection is an interesting electrochemical method applied to protect metal from corrosion. In this method, an anodic direction of the metal is administered to the passivity potential by applying an external current. The anodic protection can be considered mainly as a passivation process. Therefore, the method can only be applied to metals with a passification feature.
In anodic protection, potential and current control must be done very well. If a malfunction occurs in the system, the protected metal may be corrosion in a short time. Therefore, anodic protection is generally used to reduce the speed of corrosion in the environments of the violence.
The use of inhibitors
When the medium is added to a small amount, it is called inhibitors to reduce corrosion rate. Instead of using a corrosion-resistant but expensive material, some form is to be used to the use of cheaper materials by joining the inhibitor of the inhibitory. The use of inhibitor is the most economical solution to pit corrosion.
Surface coating
It is an economic way to create a little or very insulating obstacle between the protective coatings of the metal to protect from corrosion. Surface coverings can be divided into two groups, including metal coatings and non-metal coatings.
-
Metal Coatings
Metal Coatings:
-
- Hot dip
- Electroplating,
- Diffusion
- Done by methods such as mechanical coating.
-
Non-metal coatings
Other non-metal coatings containing paint and organic substances are used mainly for the protection of parts surfaces and improve their appearance. Paint material forms a protective film on the surface and protects this film cracking, or metal materials as long as peeling from corrosion.
As a result; Corrosion can seriously affect your products and processes. Requiring major repairs to damage, it may cause production downtime and higher costs. Pulsar offers wide range of products in which we are located in Chemical refer to our protective coatings and rust remover product category. Our product line provides strong protection against corrosion thanks to your equipment and you have taken precautions against the unexpected.
References:
http://blog.aku.edu.tr/salihpasa/files/2018/11/Korozyon.pdf
https://www.corrosionpedia.com/the-8-most-common-forms-of-metal-corrosion/2/1680
https://www.kmo.org.tr/resimler/ekler/f51288c412df764_ek.pdf
https://docplayer.biz.tr/52508456-Doc-dr-muzaffer-zeren.html
https://w3.gazi.edu.tr/~balbasi/BOLUM-7.pdf
http://web.hitit.edu.tr/dosyalar/duyurular/abdurrahmanasan@hititedutr231220166Q3C5L6O.pdf
https://www.metalurji.org.tr/dergi/dergi179/d179_3540.pdf

